
You know what’s hard? Trying to explain something you don’t understand. In medicine, the mantra while I was training was always “see one, do one, teach one”. Well, I can see the problem, but I just don’t get it.
The problem is called electrolysis. I think. Of course, it may be galvanic corrosion. Sigh. I just don’t get it.
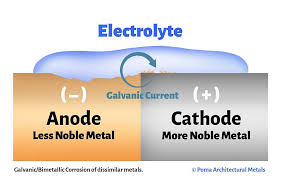
Anyway, it’s about electricity. For those of you who don’t know, electricity, most simply, is the flow of electrons from one place to another. When electrons flow from your home’s circuit panel to a light bulb, voila!, the light comes on. Well, the same thing can happen to boats, but, of course, it’s complicated. The problem with boats is that they sit in a big, big tub of salt water, which consists of sodium and chloride ions. Those ions love to conduct electrons. Which means electricity. Now, this doesn’t mean Meraviglia is glowing like a light bulb. It means she can disintegrate. Let’s say one of Meraviglia’s bilge pumps, which sit in salt water, has a wire with a hole in the insulation. Current leaks out of the hole and into the salt water. All of a sudden, there are stray electrons in the water which need somewhere to go. Without any wiring to complete a circuit, the electrons latch on to metal. Different metals have different affinities for electrons. Over time, then, electrons are stripped from some metals and move to other metals. When a metal loses electrons, it corrodes. And if the metal in question happens to be a through-hull and the through-hull corrodes and disintegrates, the boat sinks. So you see the problem. In order to prevent this, boats are equipped with anodes made of zinc or aluminum. They are usually attached to metal parts of the hull beneath the waterline and protect other metals from having their electrons stolen. Meraviglia has anodes attached to her prop shaft, rudder skeg, and prop strut. Some boats also have an anode as part of the engine.
This whole electrolysis thing made itself known to me a couple of weeks ago while I was servicing the engine. Our brand new engine has an anode as part of the seawater cooling system. However, when I took it off for inspection, it was…gone. Yep, all gone. This was, you might imagine, somewhat distressing. After that, I inspected the through-hulls below the waterline. Some of them are showing pitting and corrosion as well. Those have been in place for a year, so that isn’t terribly surprising, but for the engine anode to disappear in 4 months means electrons are a-flowin’. And not where we want them.





The way to prevent electrolysis is through a boat’s bonding system: basically one runs wire from each metal component to a metal component on the hull to allow the electrons an easy path out of the boat. There are green wires running all over Meraviglia: from chainplates, the windlass, fuel tanks, water tanks, the water heater, the prop shaft, the engine. Lots of green wires. Now, confession time: I never really understood the function of these green wires. Frankly, I’m still a bit fuzzy on it, because electricity is like magic to me. It’s there, just don’t ask me to explain it. So as we were refitting Meraviglia, I never really paid attention to the wires. In retrospect, that may have been a poor decision. Needless to say, I’m paying more attention now. Anyway, we need to figure it out so we protect our engine and our boat. I have no answer yet. We found disconnected green wires in lots of places and are going to need to figure out where they are supposed to go and get them reconnected. Also, I may have removed some which, again, may have been a poor decision.
For now, I replaced the engine anode and will be monitoring it to see how quickly it corrodes. And any of you out there who can explain stray current, galvanic corrosion, electrolysis, and marine bonding systems, please don’t hesitate to reach out. Because it’s like magic. In this case, I’m thinking black magic.

May 23, 2024 at 3:11 pm
Wow! You’re doing more electrical engineering than I’ve done! 🙂
May 24, 2024 at 8:44 pm
What I’m doing is more accurately described as electrical guessing.